FREMONT, Calif., Aug. 5, 2021 /PRNewswire/ — Verseon today announced that VE-4840 will advance as the company’s primary diabetic retinopathy (DR) development candidate after it passed preliminary toxicology studies. Orally administered VE-4840 has also demonstrated significant reduction of diabetes-induced retinal vascular permeability (RVP) in vivo.
These results, along with its excellent oral pharmacokinetics profile, support advancing VE-4840 through the remainder of IND-enabling studies toward clinical trials.
Because of the unique capabilities of Verseon’s physics- and AI-driven drug design platform, the company always generates multiple novel chemically distinct drug candidates for each disease target. The DR program is no exception: a second drug candidate, VE-3539, will also enter IND-enabling studies shortly after those for VE-4840.
DR and the frequently associated condition diabetic macular edema (DME) arise when chronically high blood sugar levels weaken retinal blood vessels. The resulting progressive vascular injury causes hemorrhage and fluid leakage into the retina that often leads to retinal thickening, blurred vision, and central vision loss. Left unchecked, it can lead to blindness.
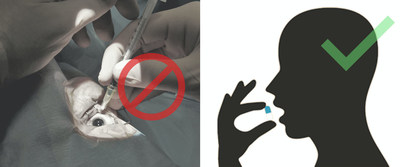
Over 154 million patients worldwide—one third of all diabetics—are currently in some stage of DR. Unfortunately, the current standard of care is regular intravitreal injections for moderate to severe disease. These treatments carry significant risk—in addition to obvious discomfort—and consequently, doctors simply monitor DR when mild. Treatment begins if growth of new blood vessels accompanies DR (called proliferative DR or PDR) or if non-proliferative DR (NPDR) becomes severe.
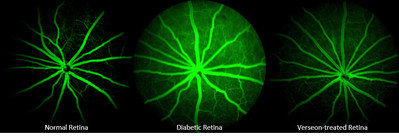
Verseon’s VE-4840 is a first-in-class potent and highly selective reversible covalent inhibitor of plasma kallikrein, a validated protein target implicated in the disease pathway of DR and DME. It could provide a much-needed alternative to more invasive treatments, allowing safe prophylactic use and treatment in all stages of disease severity.
Previous results presented at ARVO demonstrated VE-4840’s efficacy in reducing both plasma kallikrein-induced and VEGF-induced retinal thickening and vascular permeability when orally administered. Further studies conducted in-house have demonstrated that VE-4840 is efficacious in reducing diabetes-induced RVP in an in vivo rodent model, which captures key elements of human DR. Second candidate VE-3539 has yielded similar test results. To our knowledge, Verseon’s molecules are the first oral inhibitors showing efficacy in this model.
"The results of our in vivo studies are another step towards creating the first orally administered diabetic retinopathy drug needed by millions worldwide for both treatment and prophylaxis," said Verseon CEO Adityo Prakash.
About Verseon’s Diabetic Retinopathy Program
Verseon has developed a new class of potent, selective small-molecule plasma kallikrein inhibitors for the treatment of diabetic retinopathy, a major cause of adult blindness. Several lead candidates have demonstrated efficacy in reducing retinal thickening and leakage—two hallmarks of the process leading to a major form of diabetic vision loss. In contrast to current treatments administered as either recurring eye injections or implants, Verseon’s drug candidates can be orally administered as a possible monotherapy or in conjunction with current treatments for those with advanced DR. The company is currently preparing development candidate VE-4840 for Phase I trials.
About the Company
To advance global health, Verseon International Corporation (www.verseon.com) has created a better, more scalable process for designing and developing new drugs addressing currently untreatable or poorly treated conditions. The company’s drug development platform incorporates fundamental advancements in molecular modeling, directed synthesis, integrated translational research and advanced AI to develop drug compounds that have never before been synthesized—and are virtually impossible to find using conventional methods. Verseon is a clinical-stage company with a growing pipeline that currently includes seven drug programs in the areas of anticoagulation, diabetic retinopathy, hereditary angioedema, oncology, and metabolic disorders.
For media inquiries contact:
Andy Abramson
Comunicano, Inc.
+1 858-777-9777
315963@email4pr.com
SOURCE Verseon International Corp.